Boost COVID-19 vaccine R&D with rapid serology tests and minimal sample and reagent consumption (Part III)
An open platform to enable rapid data generation in a dynamic R&D environment
A review article from 2019 that, in a way, foresaw the COVID-19 pandemic by less than a year, pointed out, “In the case of emerging infectious diseases, it can be difficult to predict what assays will be most useful or informative or will perhaps even provide an immune correlate of protection for a vaccine in early development” (1). Typical methods used in vaccine R&D include enzyme-linked immunosorbent assays (ELISA), virus neutralization or bactericidal immunoassays, and ELISpot.
The assay toolbox evolves over time from early vaccine development to increase throughput, reduce sample volumes, and automation to enable increased throughput for late-stage clinical trials.One platform that has emerged as an established and valuable alternative to ELISA is the Gyrolab® platform, which offers a number of advantages when running established assays, such as for SARS-CoV-2 antibodies, and also when transferring assays, or developing new ones (Table 1).
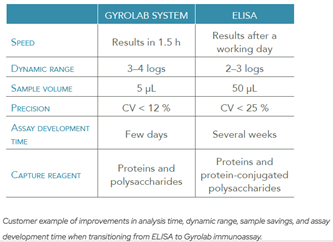
Table 1. Comparison of Gyrolab immunoassay performance to ELISA
Gyrolab SARS-CoV-2 Total Antibody Assay
In response to the COVID-19 pandemic, Gyros Protein Technologies has developed a three-step bridging serologic immunoassay test using commercially available reagents to be run on the open Gyrolab platform. The assay has a broad analytical range for qualitative detection of total antibodies generated against the spike protein (RBD) of SARS-CoV-2 in human serum samples.
The qualitative, Gyrolab SARS-CoV-2 Total Antibody Assay* can be used to detect antibodies over a broad range of concentrations, from 40 ng/mL to 200 µg/mL. Adding unlabeled RBD completely inhibited the response from positive controls, confirming the specificity of the assay, and the responses from 50 pre-pandemic serum samples were equivalent to negative controls. The open Gyrolab platform also enables this SARS-CoV-2 RBD immunoassay to be readily complemented with immunoassays directed to other targets.
Find out more by downloading the White Paper: One-hour, microfluidic SARS-CoV-2 antibody immunoassay using Sino Biological reagents and Gyrolab® immunoassay platform.
*Note that this assay has been developed for research use only and is not intended for diagnostic use.
References: