Accurate ligand affinity determination: in-solution Adnectin KD measurement
Johan Engström, Sr Scientist at Gyros Protein Technologies
Part 2: Determining the dissociation constant (KD) for a high-affinity Adnectin™ imaging ligand by in-solution affinity measurement
Analyzing high-affinity ligands used to target protein-protein interactions can be a real challenge. A biotherapeutics discovery team at Bristol Myers Squibb (BMS) used Gyrolab® in-solution affinity analysis to overcome problems in determining the picomolar-level dissociation constant of an imaging Adnectin™ ligand. Advantages also included low sample consumption, high throughput, and more accurate values for low-picomolar dissociation constants than surface plasmon resonance (SPR).
As we saw in the first post in this series, while cancer immunotherapy is an exciting and potentially game-changing therapeutic approach, success of clinical studies may require the imaging of targets, for example by using designer ligands that bind to the programmed death-ligand 1 (PD-L1) . Ligand affinity is a critical parameter, which is why Zhou Dai and colleagues at BMS (Discovery Biotherapeutics, East Coast) decided to determine the in-solution affinity of 19F-BMS-986192, an Adnectin™ binding human PD-L1 with high affinity that is in clinical trials as a positron emission tomography (PET) imaging agent (1).
Measuring picomolar dissociation constants with Gyrolab® system
BMS had only been able to estimate the dissociation constant (KD) of the PET Adnectin to <35 pM in earlier work using surface plasmon resonance (SPR). The problem was that SPR has difficulty measuring high-affinity interactions accurately because the slow dissociation rate demands long measurement times, resulting in KD’s that are close to the instrument’s detection limit. The discovery team therefore decided to measure the KD’s using in-solution affinity analysis, which works well even with extended incubation times.
In-solution affinity analysis of the imaging ligand involved mixing fixed amounts of one interactant (PET Adnectin) with a serial dilution of variable interactant (PD-L1), incubating to reach equilibrium, and using Gyrolab system to measure the amount of free, fixed interactant (PET Adnectin). The KD was calculated using Gyrolab Affinity Software module with multi-curve analysis, which enables global fitting of affinity curves collected at different fixed interactant concentrations to give a more accurate KD – see Figure 1. The discovery team at BMS also used Gyrolab system to measure the affinities of two antibodies and compared the results with KinExA (Sapidyne Instruments).
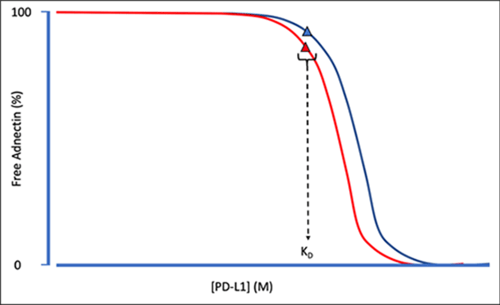
Figure 1. Schematic showing multi-curve affinity analysis for the PET Adnectin at two fixed concentrations with PD-L1 dilution series after equilibrating at room temperature for 22 h. Since several curves originating from the same interaction have the same KD value these curves can be analyzed together using Multi-Curve Analysis to improve estimates of the KD. Based on Figure 2, Dai et al, 2020.
The team obtained a dissociation constant for the PET Adnectin of 13 ± 4.7 pM, which is consistent with the value of <35 pM they had determined by SPR. They could also confirm that Gyrolab system and KinExA gave comparable affinities for the two antibodies (mAb1 KD, 52 pM and 41 pM; mAb2 KD, 37 pM and 14 pM; for Gyrolab and KinExa, respectively).
Immunotherapy – over 100 years of progress towards high-affinity ligands
Work like this shows how far we have come since the pioneering work of William Coley, the “father of cancer immunotherapy”, in the late 19th century. Frustrated by failures in cancer treatment, his observation that acute bacterial infections could lead to spontaneous remission gave him the courage to inject live bacteria into a patient with an inoperable malignant tumor to induce an immune response. Amazingly, this rather crude approach worked. Progress since then has led to increasingly refined biotherapeutics that target protein-protein interactions (PPIs) in a wide range of tumors with high precision and affinity at the picomolar level.
The Discovery Biotherapeutics team at BMS showed that Gyrolab system quickly delivered reliable affinity data that was more accurate than SPR. They also appreciated the low sample consumption (< 10 µL) and high throughput of Gyrolab system that enabled them to develop assays more quickly compared to KinExA® (Sapidyne Instruments).
You can find out more about in-solution affinity analysis on our affinity interest page, where you can also download a Technical Note on in-solution affinity determination using Gyrolab systems
References:
- Application of the Gyrolab microfluidic platform to measure picomolar affinity of a PD-L1-binding Adnectin™ radioligand for positron emission tomography. Dai Z. et al. BioTechniques 2020. 69: 00–00 10.2144/btn-2020-0080